
[ad_1]
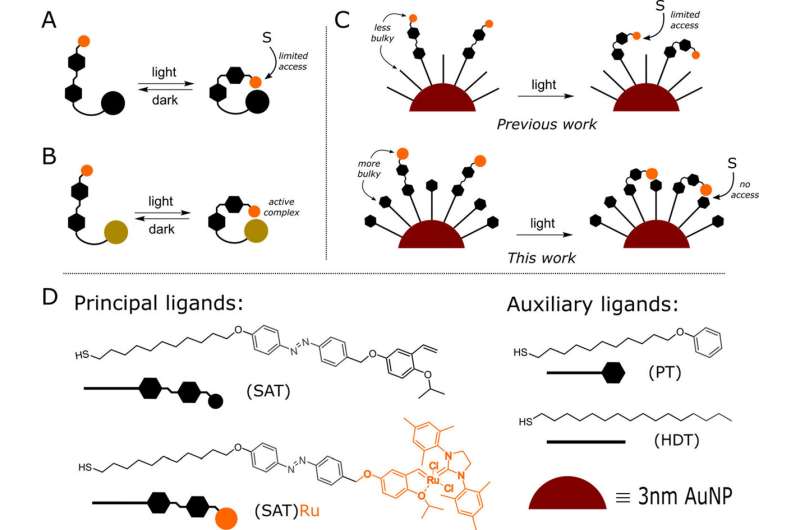
General strick approaches to control catalysis using photoswitches: (A) shielding the active site using a bulky molecular handle; (b) bringing the catalytic units together to form an active complex; (C) our approach based on regulation of monolayer bulkiness at the nanoparticle level; and (D) ligands and nanoparticles used in the present study. Credit: ACS catalysis (2023). DOI: 10.1021/acscatal.3c04435
Nature is amazing. It has developed the ability to regulate complex biochemical processes in living organisms with remarkable efficiency. Enzymes, natural catalysts, play an important role in this regulation, ensuring that various physiological needs are met throughout the cell’s lifespan.
Additionally, specific organic molecules and metal ions bind to enzymes, modulating their catalytic activity up or down. This interaction of activators and inhibitors synergistically maintains order in the cascade of chemical reactions within cells.
Enzymatic catalysis continues to inspire scientists to mimic nature to control a variety of processes in many fields, from small laboratory scales to large-scale industrial production of many chemical compounds. However, despite the high efficiency of synthetic catalysts, the alternation between acceleration and inhibition does not prevent them from working completely or is limited to the use of additional chemicals.
This limitation becomes particularly important in managing simultaneous and sequential processes, where undesirable parallel responses may persist despite change efforts. Consequently, many research efforts are underway on ways to control complex transformations in an efficient and environmentally friendly manner, while minimizing the use of additional chemicals, with particular emphasis on altering the initiation and termination of selective reactions. Is.
Is it possible? A new concept described In the journal ACS catalysis sheds a clear light on this question.
A new approach proposed by researchers from the Institute of Physical Chemistry of the Polish Academy of Sciences (IPCPAS), led by Professor Włodmyr Saszczuk, has simplified catalytic processes using light. Control has been demonstrated, which can generally be an alternative to chemical regulation. Enzymes Based on the proposed concept, it would be possible to selectively slow down or speed up chemical reactions in a completely controlled manner, without depleting the catalyst itself. How it works?
“We show that catalysis can be controlled by encapsulating the catalyst within an organic monolayer that envelopes most of the surface of the inorganic nanoparticles. Thanks to this, the complete suppression Catalytic activity can be achieved,” claims Professor Vladimir Sashuk.
The researchers focused on turning the reaction on/off using nanostructured materials, in which catalysis can be turned on or off using a specific wavelength, acting like a “light switch.” does. This material was based on ~3 nm sized gold nanoparticles (Au NPs) decorated on their surface with ruthenium-based organic N-heterocyclic carbene (NHC) complexes through strong Au-S bonding between AuNPs and thiol ligands. had gone.
The uniqueness of the proposed material lies in its structure, in which a bulky thiol (PT) provides steric hindrance, while an azobenzene-containing thiol (SAT) supports a Hoveyda-Grubbs ruthenium complex, called the precatalyst. which initiates the catalytic process. Reaction with substrate.
The designed nanosystem is sensitive to a specific range of light, allowing the percatalyst to change its position within the organic monolayer and control access to the substrate and catalysis through electromagnetic stimulation.
In the presence of visible light or in the dark, the ruthenium-based precatalyst is exposed to solution, initiating and sustaining the metathesis reaction. Conversely, when the system is subjected to ultraviolet irradiation, the azo ligand undergoes isomerization, which acts like a “push button” to inhibit percatalyst activation.
This is facilitated by a material design in which the phenyl rings of the PT ligands block access to the precatalyst, masking it from the solution and effectively inhibiting the catalytic process. The feasibility of this mechanism is supported by theoretical simulations by scientists at the University of Trieste in Italy.
Prof. Paola Pusocco further explains, “Our calculations demonstrated that the surface of a gold nanoparticle coated with phenyl moieties is better protected from incoming molecules than one containing only aliphatic chains.” occurs. Clearly, this translates to the observed catalyst shutdown.”
The proposed method allows fast and highly efficient actionable Enables deactivation and control of reaction rates without the use of additional chemicals. The researchers believe that their unconventional approach to photoinduced manipulation of the position of the precatalyst in the proposed material will help provide many active catalysts that will find applications in various fields, particularly chemical selectivity. In the enhancement circle. At the same time, they emphasize the role of interdisciplinarity during research.
More information:
Mykola Kravets et al., Pursuit of the Complete Off-State in Photoswitchable Catalysis, ACS catalysis (2023). DOI: 10.1021/acscatal.3c04435
Provided by
Polish Academy of Sciences
Reference: Using light to control catalytic processes (2024, February 23) Retrieved February 23, 2024, from https://phys.org/news/2024-02-catalytic.html.
This document is subject to copyright. No part may be reproduced without written permission, except for any fair dealing for the purpose of private study or research. The content is provided for informational purposes only.
[ad_2]


